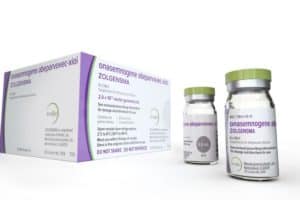
Zolgensma
Credit: Wall Street Journal
The day, long anticipated, when America’s system of voluntary drug pricing could break down has now arrived. Novartis has announced it will charge $2.1 million for Zolgensma, a gene therapy for infants with lethal spinal muscular atrophy. (emphasis added)
The seeds of this extraordinary price were sewn in 1983, when Congress passed the Orphan Drug Act, a well-meaning law designed to encourage research into rare diseases. It offered drug makers tax breaks and other incentives for such work, rapid review by the Food and Drug Administration, a lower bar for market approval, and longer protection from competition.
The law has worked so well that orphan drug research increasingly crowds out investigations into drugs for such common illnesses as heart disease and diabetes.
That’s not to say orphan drugs aren’t at times miraculous. Zolgensma, by augmenting defective DNA, changed the course of neuromuscular decline in 15 babies, enabling them to achieve developmental milestones. Nor is it to suggest that orphan diseases don’t deserve […]









his shows why we need a single-payer system like Bernie Sanders and many others suggest.
t is not a miracle if it costs so much that no one can afford it.